In the psychedelic medicine community, it can feel like all buzz with little progress towards real-life accessibility. Sure, trial results have been great for participants, but are we any closer to legally delivering therapeutic psilocybin, MDMA, or LSD to patients? This week, let’s talk about FDA approval for psychedelic substances and how soon you can expect psychedelic therapy to be available. (Image credit Mush Stock)
If you want to get involved in psychedelic medicine, now is a great time. We’re seeing massive increases in interest in these substances. People are excited about the remarkable outcomes in psychedelic trials. And we’re closer than ever to being able to administer psychedelic-assisted therapy to patients. Yet some roadblocks remain – FDA approval and drug scheduling are big ones.
Psychedelic Support is leading the charge in psychedelic-assisted therapy education. We’ve seen some medical psychedelics move through FDA approval with good results. Ketamine has showed widespread commercialization in the last decade. Medical cannabis is now available in many states.
Yet MDMA, psilocybin, and others remain scheduled substances. We believe this will change in the next few years. But before we talk about where we are in FDA approval for these substances, let’s first get clear on what the difference is between FDA approval and legalization.
Follow your Curiosity
Sign up to receive our free psychedelic courses, 45 page eBook, and special offers delivered to your inbox.FDA Approval vs. Legalization
FDA approval and legalization are very different terms, yet the difference between them can seem unclear. Let’s define them:
- Food and Drug Administration (FDA) Approval: This means a food or medication has been tested thoroughly and approved for safety. Through several large-scale studies, they’ve determined a medication’s benefits outweigh its risks or harmful effects.
In the case of medication (and substances), the FDA tests and approves them for a specific purpose. But the FDA doesn’t control how individual medical providers practice. Some clinicians may use medications to treat conditions that weren’t FDA-approved. This is called Off-Label usage.
For instance, Ketamine is an anesthetic used off-label to treat psychiatric conditions like depression. This off-label usage in a medical setting is legal. But just because the FDA approves a substance doesn’t mean it’s legalized for the general population.
- Federal Legalization: This means a psychedelic substance has been removed from the Drug Enforcement Administration’s list of scheduled substances. The DEA can choose to de-schedule a substance at any time. The Department of Health and Human Services (DHHS), drug manufacturers, and private citizens can also petition to have a substance removed.1
LSD, MDMA, and peyote are currently Schedule I substances. This means the DEA believes they have no medical benefit and a high potential for abuse. Schedule II substances have medical uses but can lead to dependency.
It’s possible that rather than de-scheduling MDMA, the DEA could move it down to Schedule II or III after FDA approval. This would mean that private use can still be prosecuted but to a lesser extent.
It’s also important to note that federal legalization doesn’t mean state-level legalization. Most states have a “trigger” system that follows DEA decisions, but others will decide for themselves whether to legalize after DEA de-scheduling.
The systems of FDA approval and legalization are totally separate. FDA approval happens through several stages of testing and data gathering. Legalization happens through actions from elected officials and democratic legalese. Once a new substance is approved, the DEA has three months to re-schedule or de-schedule.
Once the FDA approves MDMA, it may be legalized, but not necessarily. And sometimes, a substance can go without FDA approval or federal legalization and still be available on a state level. This is what we see in the case of Oregon psilocybin. More on this later. Let’s talk about the current atmosphere around FDA approval for psychedelics.
The Biden Administration & FDA Approval for Psychedelics
Current leaders have expressed support for exploring psychedelics at the federal level. In July, The Biden Administration released a statement that they anticipate FDA approval for psychedelic-assisted therapies within the next two years.2
They also indicated that the Substance Abuse and Mental Health Services Administration plans to start a task force for psychedelic commercialization. This would boost efforts to address problems standing in the way of clinical, regulatory, and public policy change.
Are We Close to FDA Approval for Psychedelics?
For some substances, yes. The psychedelics closest to FDA approval are MDMA for PTSD and psilocybin for treatment-resistant depression. Both are in late-phase clinical trials this year. This means they’ve shown safety and efficacy in small groups, and are now proving it in more diverse, larger study populations.
MDMA is rapidly progressing towards FDA approval with the help of the Multidisciplinary Association for Psychedelic Studies (MAPS). They’ve been putting together an impressive list of trials focusing on patients with chronic PTSD. Their second Phase III trial will conclude at the end of this year, and once it’s done, MAPS will apply for FDA approval. MAPS expects MDMA to be approved for PTSD late next year.
Earlier this year, we chatted with MAPS to chat about their commercialization process. You can view the talk and read the summary on our blog.
Psilocybin is close behind MDMA. In 2018, the FDA granted psilocybin a Breakthrough Therapy designation. This allowed Compass Pathways to research psilocybin-assisted therapy for treatment-resistant depression. In 2019, they gave a similar designation to the Ursona institute. These designations are a good sign for when applications for approval come around. The FDA has already shown support for psilocybin research.
This year, Compass is doing the first phase III trial ever to study psilocybin. They’ll need a second phase III trial before they can approach the FDA for approval. Given the Biden Administration’s messaging, the FDA could approve psilocybin for depression as early as 2023 to 2024.
How Soon Will Psychedelic Medicine Be Available to Patients?
You can get it now in some states. Let’s break down accessibility by substance.
- Ketamine-assisted therapy is already available in every state and can be helpful for patients with depression. It’s also a treatment for PTSD, anxiety, and other mental illnesses. But if you’re looking for ketamine-assisted therapy, beware. There’s a big difference between ketamine infusions and ketamine therapy. Read about it here.
- Psilocybin-assisted therapy will be available in Oregon starting next year. In 2020, Oregon not only decriminalized psilocybin but promised to create state-sponsored access to psilocybin sessions. This’ll make psilocybin available not only to people with existing mental illness but to people without a diagnosis who are interested in psilocybin for self-exploration.
- MDMA-assisted therapy could be available in 2023, after FDA approval. At this point, a major barrier will be the number of therapists trained to perform MDMA therapy on patients. Initially, we can expect only patients with PTSD to receive MDMA therapy, but this could change with more research.
- Other psychedelic medicines like LSD, MDMA, peyote, and ibogaine are not as far along in research. FDA approval for psilocybin and MDMA can help these lesser-researched substances gain traction for future approval.
The Latest Psilocybin Clinical Trial Update: Compass Pathways Phase III Trial Design
This week, Compass Pathways revealed the trial design for their much-anticipated Phase III program. You may recall that in November of 2021, the topline results from the phase II trial showed promising treatment effects while also highlighting the need for caution with 12 severe adverse events.
The phase II trial was the largest enrolling study of any psychedelic with 233 subjects—before this study, only 151 participants had enrolled in ANY double-blinded, placebo-controlled trial.
The recently announced phase III program plans to include nearly 1,000 subjects.
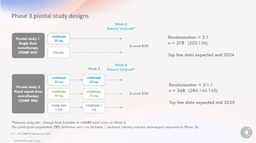
In short, there are two separate trials; the first will evaluate a single dose of 25mg vs placebo with just under 400 subjects. The second will compare two doses of three different dosages (25mg, 10mg, 1mg) in 568 subjects.
Check out this Compass Pathways press release:
“The Phase 3 program is composed of three clinical trials, two pivotal trials and one long-term follow-up, and is expected to commence by the end of 2022. The pivotal program design is as follows:
- Pivotal trial 1 (COMP 005) (n=378): a single dose (25mg) monotherapy compared with placebo. This trial is designed to replicate the treatment response seen in the Company’s Phase 2b study (n=233).
- Pivotal trial 2 (COMP 006) (n= 568): a fixed repeat dose monotherapy using three dose arms: 25mg, 10mg and 1mg. This trial is designed to investigate if a second dose can increase the number of responders and/or improve response seen in the Company’s Phase 2b study and explore the potential for a meaningful treatment response from repeat administration of COMP360 10mg.
The primary endpoint in both pivotal trials is the change from baseline in MADRS total score at Week 6.”
This latest trial design highlights how far psychedelic research has come from the small-scale, non-controlled trials of the 20th century. Compass plans to publish these findings mid-2025 with an FDA decision and commercialization in 2026 possible.
Key Takeaways About Access to Psychedelic-Assisted Therapy
There’s never been a better time to start educating yourself to become a psychedelic therapist. You could assist with ketamine therapy today or facilitate psilocybin therapy in Oregon next year. And FDA approval for MDMA and psilocybin hopefully won’t be long, either.
Perhaps the bigger question we should ask isn’t about FDA approval. Because if clinical trial outcomes keep going the way they have been, FDA approval will come. Where we see the greatest need within psychedelic medicine is for qualified, well-educated therapists.
MAPS alone aims to help 1 million patients with PTSD by 2031. To accomplish that goal, they’ll need at least 30,000 trained therapists in the next two years. FDA approval for these substances won’t matter if we don’t have psychedelic therapists to deliver therapy.
To help people access psychedelic-assisted therapy after FDA approval, we’ll need a lot more therapists to get training in facilitation and integration. If you want to become a part of the psychedelic medicine revolution, you’ve come to the right place. Psychedelic Support has resources for you, no matter where you are on your journey to becoming a psychedelic therapist.
Check out the newest addition to our free course list, Clinicians Exploring Psychedelic Therapy. This self-paced module includes terminology, cultural, and historical uses of plant medicine, dosages, risks, and more. Get three free hours of CE when you sign up and ongoing updates from our psychedelic medicine newsletter.
References
- United States Drug Enforcement Agency. (n.d.). The controlled substances act. DEA. Retrieved August 25, 2022, from https://www.dea.gov/drug-information/csa#:~:text=S.C.,The%20manufacturer%20of%20a%20drug
- Mastrine, A. (2022, July 27). Statement: Biden administration preparing for potential FDA approval of MDMA-Assisted therapy for PTSD – Multidisciplinary Association for Psychedelic Studies. MAPS. Retrieved August 25, 2022, from https://maps.org/2022/07/27/statement-biden-administration-preparing-for-potential-fda-approval-of-mdma-assisted-therapy-for-ptsd/