Patchwork Art from MAPS Therapist Training Program 2017
Photo Credit: Alyssa Gursky
Today the Journal of Psychopharmacology [1] published findings from the last study in a series of six Phase 2 trials of MDMA-assisted psychotherapy for treatment of posttraumatic stress disorder (PTSD). The MDMA therapy clinical trial in Boulder, Colorado (MP-12) enrolled 28 participants suffering from chronic PTSD. Results showed that after two to three sessions of psychotherapy with active doses of MDMA (100-125 mg) PTSD symptoms were markedly reduced. Twelve months later most participants (76%) did not meet criteria for PTSD.
After treatment, a great majority of our participants have reported feeling more connected to themselves and to others, more joy, more compassion, and with new skills for facing life’s challenges.
Marcela Ot’alora G.
Principal Investigator Boulder, CO
Decades in the Making
The Multidisciplinary Association for Psychedelic Studies (MAPS) was founded in 1986 with the intention to take MDMA through the FDA-regulated drug development pipeline. The non-profit organization faced many political and logistical hurdles to research MDMA, a schedule 1 controlled substance. In 2000, MAPS started the first randomized, blinded study investigating MDMA-assisted psychotherapy in Spain. Initial treatment of six female sexual assault survivors showed no serious adverse effects and PTSD symptoms on decline. However, the Madrid Anti-Drug Authority in 2002 [2] suddenly halted the study due to political pressure.
Marcela Ot’alora G., MA, LPC was a psychotherapist in this first study. A decade later in 2012 she became the first woman Principal Investigator for a MAPS-sponsored trial in the US. She trained and supervised nine therapy teams for the trial in Boulder, and treated 17 participants as the lead psychotherapist. Importantly, the positive results from this study showed that new therapy teams could replicate findings from previous studies (MP-1 [3], MP-8 [4]) that had a single therapy team – Michael Mithoefer, MD (Psychiatrist) and his wife Annie Mithoefer, BSN (Psychiatric Nurse). It was uncertain if interns and newly trained therapists would be able to produce significant results equivalent to the very experienced and compatible Mithoefer team.
Marcela rotated amongst teams, pairing with her husband Bruce Poulter, MPH, RN to treat several participants. Six other therapists worked on the study, including Dr. Will Van Derveer, MD, ABOIM who acted as study physician and therapist. Sara Gael Giron, MA, Director of MAPS Zendo Project, treated three participants and currently works in Boulder with Dr. Van Derveer at the Integrative Psychiatric Healing Center.
In the study publication, results were presented for 2 groups. The first was the intent-to-treat set, which refers to the group of participants who completed at least one MDMA session. The second was the per protocol set, which included only participants who completed both blinded MDMA sessions and matched all criteria for being in the study. It was discovered during the study that three participants had mental health disorders thus failing study criteria. They were excluded from the per protocol analysis. The main measure of PTSD symptom severity was change in the Clinician Administered PTSD Scale (CAPS-IV) from baseline to one month after the second blinded MDMA session. During blinded sessions, participants were given MDMA at doses of 40 mg (active placebo), 100 mg or 125 mg (active doses). Before and after MDMA sessions, participants underwent non-drug therapy sessions for preparation and integration.
PTSD Symptoms Improve
The 100 mg and 125 mg doses had a medium to strong effect on PTSD symptoms, but only the per protocol set reached significant group differences at this point. Principal Investigator Marcela Ot’alora G. commented, “The results of the study indicate that this treatment has the potential to greatly improve the lives of people suffering from PTSD, regardless of the source of their trauma”. At this point, a month after both blinded sessions, participants learned what dose group they were in. Participants given 100 and 125 mg had a third open label session, and participants given 40 mg started a new, second part of the study, or a “cross-over” where they had three open-label MDMA sessions.
PTSD symptoms, including anxiety, sleep disturbance, and depression, continued to significantly improved after the third active dose session for the 100 mg and 125 mg group. The 40 mg group showed similar reductions in symptoms after they crossed over and had three open-label sessions with 100-125 mg MDMA. In the paper, the authors describe how the treatment is effective by saying, “an enhanced therapeutic alliance combined with reduced anxiety or discomfort around difficult memories, increased self-compassion, and openness to expanding meaning of thoughts, feelings or experiences may all contribute toward therapeutic effects” [1].
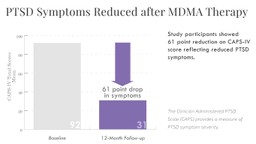
An impressive finding from this MDMA therapy study was the PTSD symptom (CAPS-IV) scores 12 months after completing treatment. Upon enrolling in the study, the average CAPS-IV score was 92, and at the 12-month follow-up, had decreased to 31. At 12-months, 76% of participants (n=25) did not meet criteria for PTSD.
“After treatment, a great majority of our participants have reported feeling more connected to themselves and to others, more joy, more compassion, and with new skills for facing life’s challenges” explained Marcela.
The average CAPS-IV (or PTSD symptom) score dropped 9.6 points from treatment exit to 12-month time point, demonstrating that MDMA therapy not only produced enduring reduction in PTSD symptoms but allowed for continued healing and growth long after the drug-therapy ended. With PTSD symptoms no longer interfering with emotional and cognitive processes, the participants were able to re-integrate into life and recover from PTSD. “What we see after reprocessing of memories and feelings,” says researcher Dr. Lisa Jerome, “is not only reduction in PTSD symptoms but improvement in other areas of their lives, including mood and sleep.”
Based on the positive safety and efficacy outcomes from this trial and the other similar Phase 2 studies, FDA granted Breakthrough Therapy Designation for this innovative treatment approach. Breakthrough Therapy is only given for drugs under development that have data indicating that they work better and/or more safe than current medications for a life-threatening illness. This designation is intended to speed up timelines in the path to FDA approval.
Though originally planned to start earlier, Phase 3 trials will start in November 2018 due to unforeseen delays with producing and encapsulating Good Manufacturing Practices (GMP) MDMA. If two trials have significant outcomes in 200-300 participants, MDMA-assisted psychotherapy could be an FDA-approved treatment by 2021.
What we see after reprocessing of memories and feelings is not only reduction in PTSD symptoms but improvement in other areas of their lives, including mood and sleep.
Dr. Lisa Jerome
MAPS PBC Researcher
In preparation for Phase 3 trials in the USA, Canada, Israel, and Europe, the MAPS Therapy Training Program has expanded to meet the need for well-trained therapists at new study sites. Therapists in this program are trained on the MDMA Treatment Manual, which was first authored by Michael Mithoefer and updated regularly as the therapeutic approach is fine-tuned.
Marcela is a lead trainer, along with the Mithoefers, and brings her passion for art to the training program. She teaches trainees about how art can be used as a non-verbal expression of internal processes and offers ways to bring art into therapy sessions during and after altered states of consciousness.
The Phase 3 MAPS Therapy Training Program consists of online e-learning modules, reading assignments, a 7-day in-person training to watch and discuss MDMA psychotherapy videos from completed studies, a 5-day in-person meeting for team building and role play, and supervision during an open-label Phase 2 MDMA study. The program will continue to scale and adapt to accommodate more trainees interested in providing MDMA therapy in Expanded Access and post-approval psychedelic clinics.
Boulder, CO will be one of approximately 15 study sites in the Phase 3 trials. Enrollment of new participants will start in November 2018. Visit maps.org for more information about study participation, therapist training, and research updates.
References
- Ot’alora, G., Marcela, Jim Grigsby, Bruce Poulter, Joseph William Van Derveer III, Sara Gael Giron, Lisa Jerome, Allison A. Feduccia, Scott Hamilton, Berra Yazar-Klosinski, Amy Emerson, Michael C. Mithoefer, Rick Doblin. “MDMA-assisted psychotherapy for Treatment of Chronic Posttraumatic Stress Disorder: A Randomized Phase 2 Controlled Trial.” Journal of Psychopharmacology. (2018). Full article link.
- Bouso, José Carlos, Rick Doblin, Magí Farré, Miguel Ángel Alcázar, and Gregorio Gómez-Jarabo. “MDMA-assisted psychotherapy using low doses in a small sample of women with chronic posttraumatic stress disorder.” Journal of Psychoactive Drugs. 40, no. 3 (2008): 225-236.
- Mithoefer, Michael C., Mark T. Wagner, Ann T. Mithoefer, Lisa Jerome, and Rick Doblin. “The safety and efficacy of±3, 4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study.” Journal of Psychopharmacology. 25, no. 4 (2011): 439-452.
- Mithoefer, Michael C., Ann T. Mithoefer, Allison A. Feduccia, Lisa Jerome, Mark Wagner, Joy Wymer, Julie Holland et al. “3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial.” The Lancet Psychiatry. 5, no. 6 (2018): 486-497.